That metabolic switch involves a change to the way in which cells produce energy, transitioning from a process called glycolysis to one called oxidative phosphorylation.
Earlier studies showed that a metabolic compound called succinate, produced by cells, can accumulate in the heart when it’s deprived of oxygen, such as during a heart attack. This can trigger a cascade of events that leads to the production of harmful molecules, called reactive oxygen species, that can damage heart cells.
Previous studies also showed that blocking another cellular compound called succinate dehydrogenase could prevent the accumulation of succinate and subsequent damage. Inhibiting the compound can also prompt a metabolic shift in cells to glycolysis, which promotes heart regeneration.
Mahmoud’s team investigated whether they could alter this metabolic switch and preserve the ability of the heart to regenerate following damage. First, they focused on succinate, which in newborn mice reduced the regeneration of new heart cells and caused DNA damage following myocardial infarction.
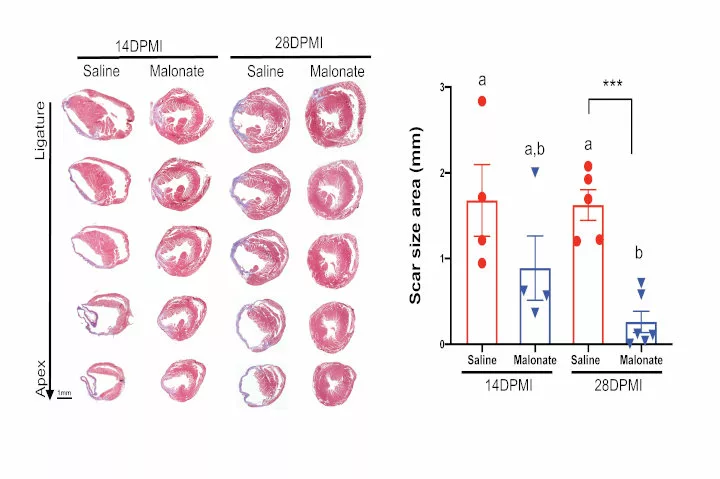
Then, in young mice, the research team used a metabolite called malonate, which blocks succinate dehydrogenase, to see if it would preserve the capacity of heart cells to regenerate following a heart attack. The treatment not only resulted in complete heart regeneration, it also restored heart function.
To ensure the effects were due to inhibition of succinate dehydrogenase, the research team used another inhibitor, a compound called Atpenin A5, and found effects similar to that produced by malonate.
In adult mice, the researchers also tested whether malonate could, as in young mice, restore the ability of the heart to regenerate following myocardial infarction. They found that it promoted the production of new heart cells, helped bring new blood vessels into the region damaged by the infarction, and led to heart regeneration. It also reinforced the finding that succinate dehydrogenase helps the heart switch from oxidative phosphorylation to glycolysis.
“Both vascular and myocardial damage arise from acute cardiovascular events such as myocardial infarction,” Mahmoud explains. “The limited capacity of the adult heart to repair itself represents a major barrier in cardiovascular medicine and often leads to heart failure. Our research shows that it may be possible to improve the function of the heart muscle after infarction, which is good news for people who have systolic heart failure.”