Stem cells could help cancer patients fight dangerous infections
Scientists at the University of Wisconsin–Madison have developed a more efficient way to grow the white blood cells, which serve as front-line defenders against bacterial infections but are often depleted as a potentially deadly side effect of cancer treatment.
Chemotherapy can leave cancer patients with a very low number of a specific type of white blood cell called neutrophil granulocytes, resulting in febrile neutropenia, a dangerous condition marked by fever and heightened risk of infection.
The condition is often treated with a transfusion of these white blood cells from a donor. But collecting enough granulocytes for transfusion is difficult, according to Igor Slukvin, MD, PhD, professor of pathology and laboratory medicine at the UW School of Medicine and Public Health, and the transfusions don’t always show the intended benefit in controlled trials.

“The complicated logistics of granulocyte collection, the need for pre-treating donors with G-CSF [a treatment that stimulates bone marrow to produce granulocytes] or steroids, difficulties in collecting a sufficient number of good quality granulocytes and the limited storage time of around 24 hours all hamper the utility of granulocyte transfusion for correcting neutropenia and may contribute to the inconclusive results observed in clinical trials,” he said.
In 2009, Slukvin’s lab pioneered transforming stem cells into multiple types of white blood cells. Now, with collaborators including Anna Huttenlocher, MD, professor of pediatrics and medical microbiology and immunology, and support from the National Institutes of Health and a UW2020 grant, the researchers have developed a method to prompt induced pluripotent stem cells to differentiate into a bed of granulocyte progenitors that can continuously produce the valuable neutrophils for weeks.

The technique, published recently in the journal Stem Cell Reports, refines the process of goading the stem cells to mature into neutrophil producers by replacing the standard expensive, relatively inefficient and time-intensive process using a series of small molecules called cytokines that carry signals from cell to cell.
Instead, the researchers introduce a piece of modified messenger RNA that sparks the production of an ETV2 transcription factor – a protein that binds to DNA, controlling the flow of genetic information – that guides the stem cell down the blood developmental path.
The stem cells become a sheet of hemogenic endothelium that begins differentiating into pure neutrophils. The white blood cells float away, and can eventually be collected and administered to patients without some of the risk caused by other blood products often carried along in transfusions.The new procedure produces neutrophils in as soon as 14 days, compared to as much as a month in previous studies, and can generate up to 17,000,000 neutrophils from 1,000,000 human induced pluripotent stem cells.
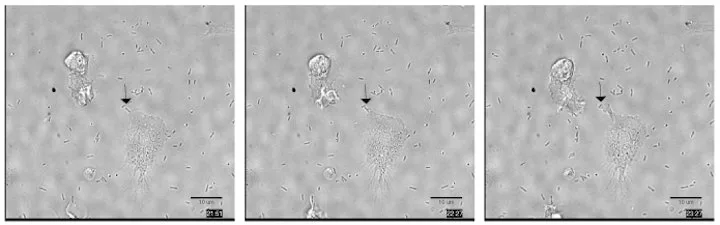
“Importantly, we found that neutrophils generated from pluripotent stem cells using this approach are functionally similar to peripheral blood neutrophils,” Huttenlocher said. “They can phagocytize (surround and swallow) and kill bacteria.”
The neutrophils also afford the opportunity to study disease.
White blood cells produced from stem cells carrying genetic disorders that weaken or otherwise affect the neutrophils would retain those problems. The new production method for stem cell-sourced neutrophils could give researchers like Huttenlocher – who studies the way white blood cells move around the body and interact in diseased and damaged tissue – a ready source of malfunctioning cells and the chance to observe them in their earliest stages of development.
In addition to Slukvin and Huttenlocher, authors of the new report include Vera Brok-Volchanskaya, formerly a Wisconsin National Primate Research Center researcher, Kran Suknuntha, postdoctoral researcher in pathology and laboratory medicine, and researcher David Bennin and graduate student Lucas Klemm in pediatrics.
This work was supported by grants from the National Institutes of Health (R01AI134749-01, R01HL142665 and P51 OD011106).