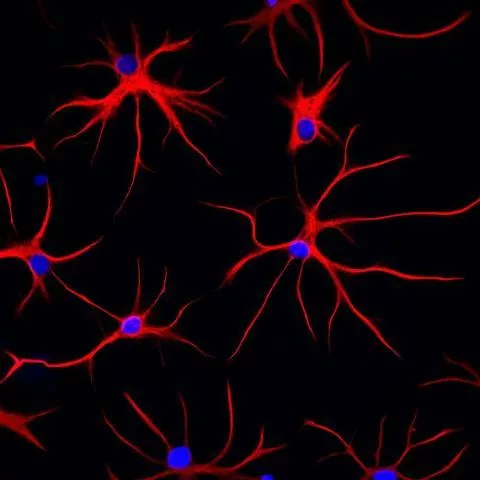
Once grown in a lab dish, the astrocytes showed the hallmarks of Alexander, including tangles built of a protein called GFAP, and errant locations of mitochondria and other cellular processing units.
GFAP, or glial fibrillary acidic protein, is a cytoskeleton protein, giving the astrocyte its distinctive star-like shape.
When the stem cells that were the source of the astrocytes were corrected with gene editing, the astrocytes subsequently derived from the engineered stem cells showed no signs of Alexander disease.
After decades of emphasizing the role of neurons, astrocytes and other glial cells are coming into greater focus for their essential contributions to the health of neurons – and for their role in disease.
Clues from rare diseases
Alexander disease itself is, fortunately, extremely rare, says Zhang, but rare diseases matter in neuroscience. “We often grow to understand a disease process through rare diseases. One mutation discovered in a family with Lou Gehrig’s disease led to the discovery of the fundamental pathogenesis of ALS, and the same is true for Parkinson’s and Alzheimer’s.”
Through studies by co-author Albee Messing, a professor emeritus of comparative biosciences, and others at UW–Madison, Alexander has been linked to a mutation in the GFAP gene, “which encodes for a protein that is very, very common in astrocytes,” Zhang says.
GFAP is so common, in fact, that astrocytes are identified through its presence, Zhang says. “GFAP was a big player for some reason, but nobody knew the broad range of effects, up until today.”
The study, published in Cell Reports, describes how Zhang, working with first author Jeffrey Jones, now a postdoctoral fellow at the Salk Institute, and others, used cell cultures to track down the role of the GFAP mutation in Alexander disease.
Alexander disease, a decades-long focus of Messing’s research, provided an ideal keyhole to study the most common protein in astrocytes, Zhang says, “but after 20 years, we still had not figured out how the mutant GFAP caused this fatal disease.”
Although common in astrocytes, GFAP is not present in other cell types.
The damage seems to start with aggregated filaments inside the astrocytes that cause widespread tangles and likely trigger a broad disturbance in cellular subunits that produce proteins, process energy and store chemicals. “The organelles — the mitochondria, endoplasmic reticulum and lysosomes — were all distributed abnormally,” Zhang says, “and that was a clue that GFAP is critical for guiding organelles to their correct locations.”