Convalescent plasma therapy has been studied during the coronavirus outbreaks that caused the 2003 SARS-CoV-1 epidemic and the 2012 MERS-CoV epidemic. It was also used during the 2009-2010 H1N1 influenza virus pandemic. Based on those studies, and some limited data published recently by doctors in China, Hartman and others believe convalescent plasma has the potential to lessen the severity or shorten the length of illness caused by SARS-CoV-2.
Earlier this month, the Food and Drug Administration expedited the compassionate use of convalescent plasma, an unapproved treatment, for the care of patients with severe and life-threatening illness caused by COVID-19. Hartman and his team have worked around the clock to begin the clinical trial, which under normal circumstances would have taken many months to bring to bear.
“Bringing this concept to reality in such a short amount of time required a Herculean effort by a team of extremely talented and dedicated people across this institution and others,” says Betsy Nugent, chief clinical research officer at the UW School of Medicine and Public Health and UW Health. “The fact that this research team was able to complete approximately 10 months of work in less than a week to get this project off the ground says a lot about their dedication and compassion and highlights this institution’s commitment to lead the development of innovative clinical treatments.”
Rapidly developing and launching the COVID-19 clinical trial required close collaboration between the School of Medicine and Public Health, UW Health, the UW Institute for Clinical and Translational Research, and local partners including Exact Sciences, which is providing the COVID-19 tests required of all potential plasma donors, and the local offices of the American Red Cross, which is extracting, processing and delivering the plasma to UW Hospital. Green Cab and Zerology will provide donors with free transportation for testing and plasma donation.
The trial came together despite the fact that many of the people involved are now working off-site due to the pandemic, says Allan Brasier, executive director of the Institute for Clinical and Translational Research. “We have been able to focus on rapidly enabling this study while ensuring the safety of our participants, providers and researchers remains paramount.”
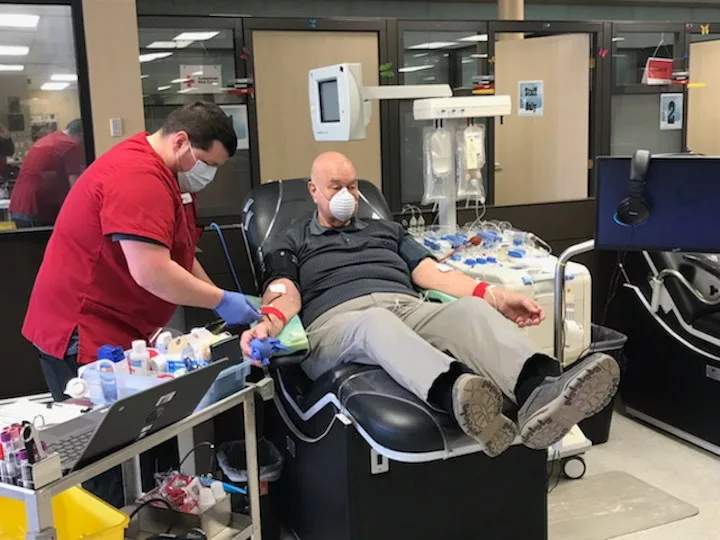
As more people recover from COVID-19 in Wisconsin, more people will become eligible to participate. Potential donors must have received a confirmed diagnosis of COVID-19, must be symptom free for at least 14 days, and will need to be tested again to confirm they are now negative for COVID-19. Those deemed eligible will be directly assigned to a specific patient in need. The experimental treatment is intended as a last resort for patients who are very ill, and they must be referred by their doctor.
People who have recovered from a confirmed diagnosis of COVID-19 and would like to donate their plasma for use in this experimental treatment can learn more by calling the Red Cross about donation 1-800-733-2767. Doctors treating COVID-19 cases at any hospital can register their patients at uscovidplasma.org.